|
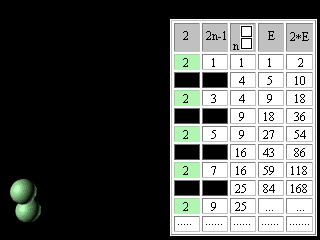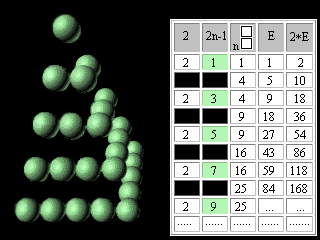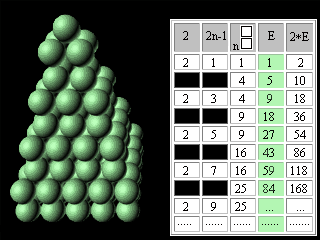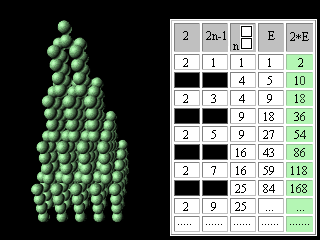
Let's introduce the second change into our modified Pascal's Triangle. Let's move apart lines of the table and in the third column above each square we will write the same square. The fourth column we will fill according to the rule of Pascal's Triangle construction. In the fifth column we will enter double numbers of the fourth column.
Figure 10. The second updating of the Pascal's Triangle. It is necessary to notice, that in the fifth column the charges of atoms ofinert, or noble, gases are written. In the fourth column, accordingly, - there are numbers of electronic pairs in these atoms, or magic numbers for shell atomic model. It was noted earlier that the Pascal's Triangle is an infinite table. May be the Mendeleyev's table has no end too? Scientists have for long been predicting the existence of superheavy stable elements. Glenn Seaborg, a recently deceased Nobel laureate chemist, one of the earliest and most outspoken advocates of experiments to reach the predicted Island of Stability, proposed the periodic table which contained 168 elements. Unfortunately,. G. Seaborg did not see the confirmation of his theory by Russian and American scientists. Element 114 was reported informally in January 1999 following experiments towards the end of December 1998 involving scientists at Dubna (Joint Institute for Nuclear Research) in Russia. Only one atom was identified and the claim has not yet been ratified, and its relatively long half-life (all of 30 seconds!) confirms predictions that a new "island of (relative) stability" may be reachable in the near future. Three atoms of element 118 were synthesizesed in experiments conducted at The Lawrence Berkeley National Laboratory, Berkeley, (California, USA), The University of Calfornia (USA), and Oregon State University, Corvallis (USA). Within less than a millisecond after its creation, the element 118 nucleus decays by emitting an alpha particle, leaving behind an isotope of element 116. This daughter, element 116, is also radioactive, alpha-decaying to an isotope of element 114. The chain of successive alpha decays continues until at least element 106. Elements 112, 110, 108 and 106 are known, but the isotopes observed in the present experiment have never before been seen. http://user88.lbl.gov/element118.html http://www.webelements.com/webelements.html At the first sight it seems, that without substantial education in the field of chemistry and nuclear physics it is impossible to understand reasonings of outstanding scientists. However, the approach even with simple (if not to say primitive) intuitively understandable positions of symmetry makes Seaborg's reasoning obvious for anyone who is able to play with cubes (or balls).
|