|
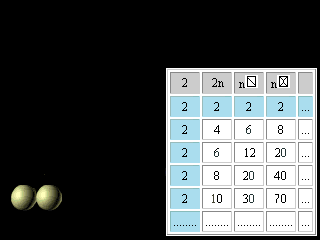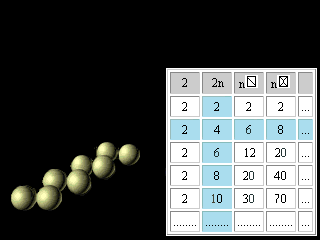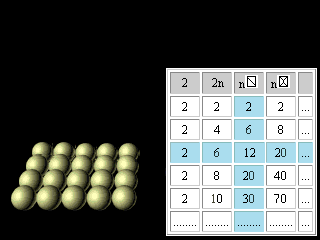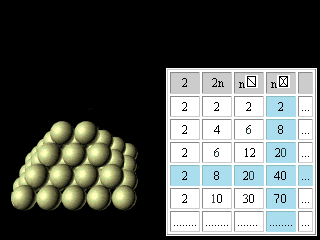
Let's now turn to regularities reflected in the right half of figures 11 and 12. The discovery and application of the nuclear shell model, for which Maria Goeppert Mayer received the Nobel Prize in 1963, together with Jensen, was one of the most important developments in nuclear physics. The magic numbers for nuclei are 2, 8, 20, 28, 50, 82, and 126, corresponding to the total number of protons or neutrons in filled nuclear shells. Thus, tin (atomic number 50), with 50 protons in its nucleus, has 10 stable isotopes, whereas indium (atomic number 49) and antimony (atomic number 51) have only 2 stable isotopes apiece. The doubly magic alpha particle, or helium-4 nucleus, composed of two protons and two neutrons, is very stable. The magic numbers are the nuclear equivalent of the atomic numbers of the noble (or inert) gases 2, 10, 18, 36, 54, 86. Nuclei with magic numbers of protons and neutrons are unusually stable and have especially large gaps between their ground and excited states.(Britannica) For magic numbers 2, 8, 20, 28, 50, 82, 126 the geometrical image was offered and the analytical formulas were found like it was made for electron atom shells. n n
Figure 23. The third updating of the Pascal's Triangle
|